Abstract

Introduction: Increased levels of fetal hemoglobin (HbF; encoded by γ-globin [HBG1/HBG2]) are protective against complications of sickle cell disease (SCD). BCL11A is a transcription factor that binds to HBG1/HBG2 promoters and produces a γ-to-β globin (fetal-to-adult hemoglobin) switch. Inhibiting BCL11A binding to its target can reverse the switch and induce HbF expression in adult red blood cells (RBCs). OTQ923 is an autologous, ex vivo, CRISPR/Cas9-edited, CD34+ cellular product with a targeted disruption of the HBG1/HBG2 promoters on chromosome 11. Targeting this region in preclinical models led to HbF induction, phenotypically recapitulating hereditary persistence of fetal hemoglobin (a naturally occurring condition whose co-inheritance ameliorates SCD severity). This approach is more targeted than complete elimination of BCL11A expression in erythrocyte precursors for treating hemoglobinopathies. Additionally, unlike lentiviral vector gene addition, induction of endogenous γ-globin transcription could concur with reduction of βS-globin expression to avoid α- and β-like globin chain imbalance.
Methods: Subjects with severe SCD, defined by a history of stroke, recurrent vaso-occlusive events (VOE), acute chest syndrome (ACS), chronic transfusions, recurrent priapism or red cell alloimmunization, were eligible to participate in the adult cohort (≥18-≤40 years old) of this clinical trial (NCT04443907). OTQ923 was manufactured from cryopreserved, peripheral blood derived CD34+ cells, collected after plerixafor mobilization. Prior to infusion, participants received myeloablative busulfan. Primary endpoints are to evaluate engraftment, long-term safety, and efficacy of HbF expression.
Results: As of the data cut-off (July 8, 2022), 2 participants had received OTQ923, with follow-ups of 9 months (mos) and 3 mos, respectively. Here, we present initial clinical data from these 2 participants; additional data will be presented at the meeting. Both participants were homozygous for the sickle mutation. Participant 1 is a 22-year-old male with a history of silent cerebral infarcts, ACS, and priapism. At study entry, he was receiving chronic transfusions. He received 2.8x106 CD34+ cells/kg of OTQ923 (2 combined manufacturing batches). Participant 2 is a 21-year-old male with a history of ACS, and a silent cerebral infarct and was receiving hydroxyurea. He received 5.99x106 CD34+ cells/kg of OTQ923 (3 combined manufacturing batches).
Time to neutrophil engraftment (the first of 3 consecutive days of absolute neutrophil count above 500 cells/mL after cell infusion) was 26 and 20 days for Participants 1 and 2, respectively. No OTQ923-related adverse events (AEs) were reported in either participant. All observed AEs were consistent with busulfan conditioning. Participants received RBC transfusions around conditioning and have received none after engraftment.
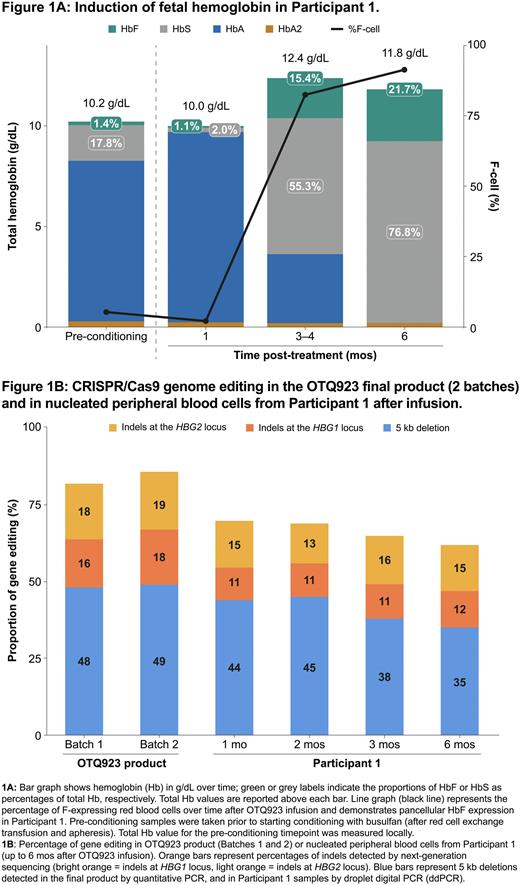
For Participant 1, HbF level increased in the 6 mos after infusion and was stable at data cut-off (22.1% HbF at 9 mos after infusion). Induction of HbF was pancellular, with an F-cell percentage around 91% at 6 mos after infusion (Fig. 1A). Participant 2 had achieved an HbF level of 15.9% at 3 mos after infusion, the latest evaluable timepoint at data cut-off. Neither participant had any SCD-related VOEs after infusion of the cellular product. The proportion of edited alleles in the peripheral blood of Participant 1 was maintained at over 60% until Mo 6, which was the last evaluable timepoint (Fig. 1B). The indels were diverse, except for a predominant, large 5 kb deletion arising from simultaneous double-stranded DNA cleavage at HBG1 and HBG2 on-target sites, similar to previous reports.
Conclusion: Preliminary data demonstrate clinically meaningful increases in HbF and evidence of benefit for both Participants 1 and 2 after OTQ923 infusion. Edited alleles were stable and maintained in the peripheral blood for >6 mos. The safety profile of OTQ923 was as expected after myeloablative busulfan and autologous hematopoietic stem cell transplantation. Taken together, these data suggest that this approach is safe and offers a potentially curative option for patients with severe SCD. Using cryopreserved, CD34+ cells as a starting material offers the advantage of centralizing manufacturing, scaling up the process to allow for improved patient access around the globe.
Disclosures
Sharma:CRISPR Therapeutics: Research Funding; Vindico Medical Education: Honoraria; Medexus Inc: Consultancy; Vertex Pharmaceuticals/CRISPR Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Other; Novartis: Other: Other; Spotlight Therapeutics: Consultancy; Magenta Therapeutics: Other: Research collaboration. Boelens:BlueRock: Consultancy, Honoraria; Avrobio: Consultancy, Honoraria; Race Oncology: Consultancy, Honoraria; Medexus: Consultancy, Honoraria; Omeros: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Advanced Clinical: Honoraria, Other: data safety monitoring board; Equillium: Consultancy, Honoraria; Sobi: Consultancy, Honoraria; SmartImmune: Consultancy, Honoraria. Hankins:GBT: Consultancy; Forma Therapeutics: Consultancy; CVS Health: Consultancy; NHLBI: Membership on an entity's Board of Directors or advisory committees, Research Funding; CDC: Research Funding; HRSA: Research Funding; ASH: Membership on an entity's Board of Directors or advisory committees. Bhad:Novartis Pharmaceuticals: Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months; Novartis Institutes for BioMedical Research: Current Employment. Lewandowski:Novartis Pharmaceuticals: Current equity holder in publicly-traded company; Novartis Institutes for BioMedical Research: Current Employment. Zhao:Novartis Institutes for BioMedical Research: Current Employment; Merck: Divested equity in a private or publicly-traded company in the past 24 months; Pfizer: Divested equity in a private or publicly-traded company in the past 24 months; Regeneron Pharmaceuticals: Divested equity in a private or publicly-traded company in the past 24 months; Johnson & Johnson: Current equity holder in publicly-traded company; Novartis Pharmaceuticals: Current equity holder in publicly-traded company; Abbvie: Divested equity in a private or publicly-traded company in the past 24 months; Bristol Myers Squibb: Divested equity in a private or publicly-traded company in the past 24 months; Editas Medicine: Divested equity in a private or publicly-traded company in the past 24 months; Intellia Therapeutics: Divested equity in a private or publicly-traded company in the past 24 months. Chitnis:Novartis Institutes for BioMedical Research: Current Employment. Ciceri:Kite Pharma: Consultancy. Yuan:Novartis Institutes for BioMedical Research: Current Employment; Novartis Pharmaceuticals: Current equity holder in private company. Yu:Novartis Pharmaceuticals: Current equity holder in publicly-traded company, Ended employment in the past 24 months; Mersana Therapeutics: Current Employment, Current equity holder in publicly-traded company. Stevenson:Novartis Pharmaceuticals: Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months; Novartis Institutes for BioMedical Research: Current Employment. De Vita:Novartis Institutes for BioMedical Research: Current Employment; Novartis Pharmaceuticals: Current holder of stock options in a privately-held company. LaBelle:AbbVie: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal